Solar-Thermal Ammonia Production
A greener and more renewable pathway for ammonia production
Ammonia (NH3) is an energy-dense chemical and vital component of fertilizer and other chemical commodities. NH3 is currently synthesized via the hydrocarbon intensive Haber-Bosch (H-B) process at high pressures of 150-300 bar and moderate temperatures of 350-500 °C. Hydrocarbons provide the heat and mechanical work required to drive this process and are also the source of the starting materials essential to the reaction.
As a result, the H-B process accounts for ~1.4% of total global CO2 emissions. The development of a low pressure renewable pathway to NH3 synthesis that uses concentrated solar irradiation for the process heat instead of hydrocarbon combustion could significantly decrease or potentially eliminate greenhouse gas emissions in the chemical production of this commodity chemical. It could also avoid the cost, complexity, and safety issues inherent in high-pressure processes.

A process developed by researchers at Sandia National Laboratories in collaboration with Arizona State University and the Georgia Institute of Technology have developed an advanced solar thermochemical looping technology to produce and store N2 from air for the subsequent production of NH3 via a two-stage process which uses recyclable metal oxide and metal nitride materials to perform the chemistry. The net result is NH3 produced from sunlight, air, and green H2.
The aim of the Solar-Thermal Ammonia Production (STAP) project is to demonstrate a sustainable pathway for NH3 production that uses concentrated solar irradiation in place of the hydrocarbon resource and decreases pressure requirements by about one order of magnitude compared to H-B.
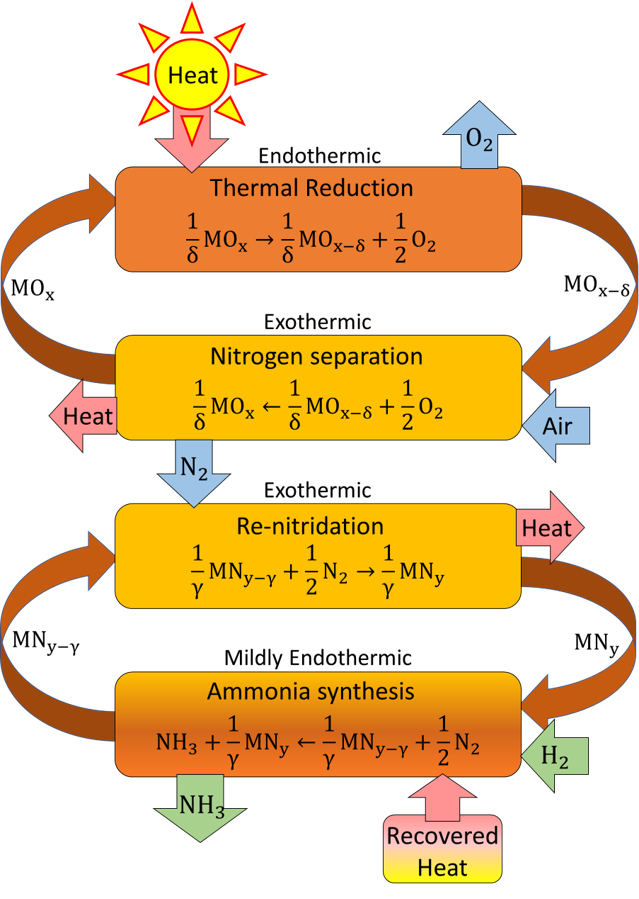
The Solar-Thermal Ammonia Production (STAP) process consists of two cycles. The solar N2 purification process (Cycle 1) is driven by concentrated solar irradiation. In the first step, endothermic thermal reduction of redox-active metal oxide particles removes oxygen from the material. In the second step, exposure to air re-oxidizes the particles by absorbing O2, resulting in relatively pure N2 gas. N2 serves as a feedstock for renewable NH3 production (Cycle 2) in an advanced two-step, low pressure, looping process. In the first step of Cycle 2, H2 reacts with metal nitride particles to produce NH3, resulting in a reduced (nitrogen deficient) metal nitride. In the second step, the nitride is regenerated using the nitrogen produced in the air separation cycle. The net result is NH3 produced from sunlight, air, and (green) H2 while the metal oxide and nitride particles are recycled.
- NH3 production via a renewable, carbon-free technology
- Inputs are sunlight, air, and hydrogen; the output is ammonia
- Significantly lower pressures than Haber-Bosch
- The process consumes neither the oxide nor the nitride particles, which actively participate cyclically
- Fertilizers
- Fine chemicals
- Concentrating solar technologies
- Transportation/fuels
- End-user for renewable H2
SD 15033.1
Published5/23/2022
Last Updated1/17/2024
