RAPTR N95: Rapidly Producible, Reusable N95 Respirator
Sandia National Laboratories in collaboration with Mayo Clinic has developed a rapidly producible, reusable N95 respirator mask designed for medical use

Significant shortages in the supply and availability of medical-grade personal protective equipment (PPE), specifically N95 respirators, have been a defining feature of the COVID-19 pandemic. Access to inexpensive, reusable, and medical grade respirators is critical to our nation’s security and public health.
For many years disposable N95 respirators, also known as filtering facepiece respirators, have been the primary PPE used by hospitals to guard staff and patients against exposure to respiratory pathogens. The COVID-19 pandemic exposed limitations in this widespread approach, with peak demand resulting in critical PPE shortages. These circumstances combined with the marketing of counterfeit disposable N95s with inferior performance have emphasized the need for rapid domestic production of trusted medical grade PPE. Despite the supply challenges, waste, and limited availability, disposable PPE remains the primary form of respiratory protection used by healthcare practitioners. Existing alternatives are frequently unable to meet requirements for use in a medical setting. Additionally, commercially available reusable respirators rely on proprietary filter media which has been subject to supply shortages during the pandemic. The challenges presented by the COVID-19 pandemic along with the inevitability of future zoonotic coronaviruses underscores the need for a readily available and low-cost respirator for medical use.
A collaboration between Mayo Clinic and Sandia National Laboratories has resulted
in the development of a rapidly producible, reusable medical grade respirator. The RAPTR
N95 was designed to address the needs of the medical community and lessen the impact
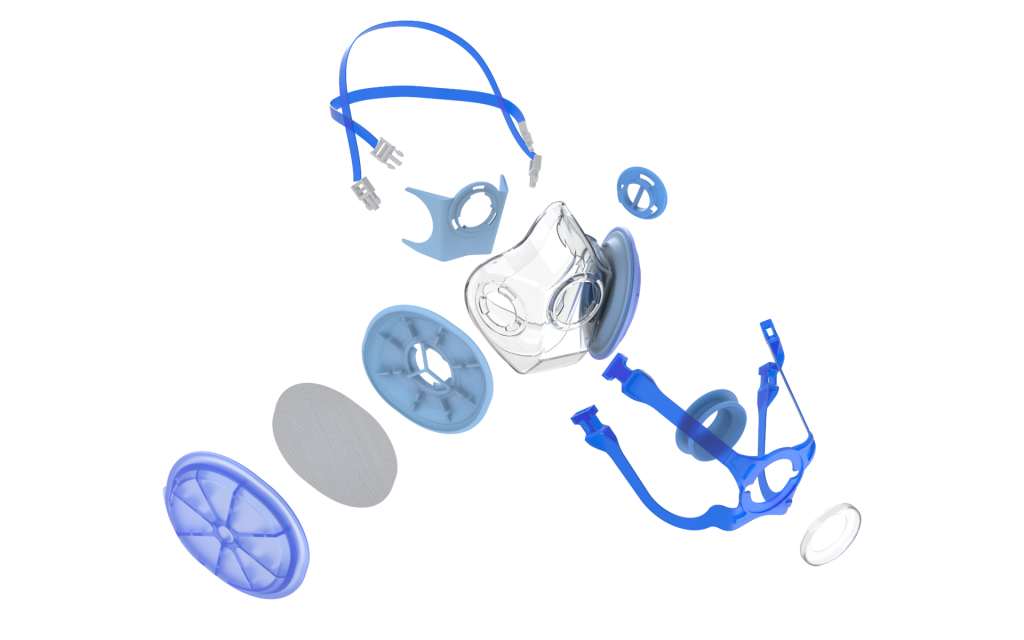
of future pandemics. The respirator’s unique design is intended to enable quick and complete
disassembly for sterilization, decontamination, and replacement. The RAPTR respirator body
is composed of a soft structure intended for prolonged use and comfort for the wearer. Air is filtered during both inhalation and exhalation to prevent risk of infection to both the wearer and to those in proximity — a requirement for most medical environments. The respirator also employs a passive resonator to transmit the wearers’ voice which can be replaced with a
port for fit certification.
The RAPTR N95 is designed to use commonly available injection molding techniques and practices for rapid production. For this reason, the design can be produced within regional manufacturing networks to improve access to medical grade N95 respirators for regular or emergency use. Features such as the mask’s filter geometry, filtration during inhalation and exhalation, easily replaceable fit test port, and full disassembly of all components reduces the cost to manufacture and maintenance costs for the end user.
The RAPTR N95 provides unique and high-end features at a low cost. The RAPTR N95 is intended to reduce waste and improves reliability compared to disposable N95s . Most notably, the RAPTR N95 reduces supply chain dependency on proprietary filters by allowing the use of bulk filtration media. Non-proprietary user selectable filtration material can be purchased in rolls and cut to the shape of the filter holder. Additionally, the filter media is held in a protected structure to prevent contamination and damage from biological and chemical hazards that arise in medical settings, prolonging its usability.
To date, the RAPTR N95 has undergone quantitative fit testing on multiple wearers using Occupational Safety and Health Administration (OSHA) required procedures on prototype respirators. As a medical grade reusable respirator, the RAPTR N95 can reduce the cost of consumable PPE in healthcare delivery settings and meet the needs of researchers who operate in biosafety levels 2 or 3 (BSL-2 or BSL-3) environments.
- R&D100 Award Recipient for 2021 (Analytical/Test Category)
- R&D100 Silver Recipient: Corporate Social Responsibility Special Recognition
- Reusable respirator could ease COVID-19 medical mask shortages Sandia media release (April 2021)

SD 15584, SD 15904