Low Cost TiO2 Nanoparticles
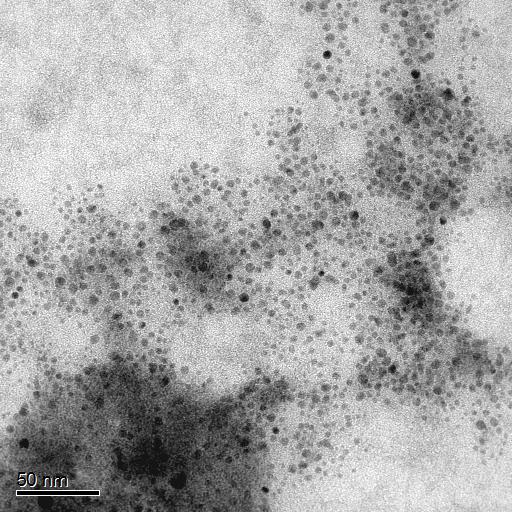
Current methods of producing titanium dioxide nanoparticles require costly surfactants and/or high temperature and pressure processing. Processing under these conditions results in nanoparticles with extremely wide particle size distributions and significant particle agglomeration. These problems are the primary reasons why TiO2 has had such difficulty transitioning from the laboratory to the marketplace. However, discrete, uniform TiO2 nanoparticles show greatpotential in numerous markets, including lighting, signage, automotive and solar energy for their excellent conversion efficiency and increased brightness. The large refractive index of TiO2 makes the material ideal for use in nanocomposites.
Sandia has developed an elegant and economically advantageous method to synthesize titanium dioxide nanoparticles. The synthesis requires only three inexpensive and commercially available reagents: titanium isopropoxide, isopropanol and water. The nanoparticles are synthesized at room temperature and ambient pressure in less than 24 hours. The nanoparticles produced are 5 nanometers in size with a narrow size distribution. They are discrete (non-aggregated) and stable in solution. In addition, these particles can be surface-functionalized to suit a wide variety of needs.
- Cost effective: Uses readily available, low cost surfactants and solvents
- Easy scale-up: Process can easily be introduced to large-scale industrial applications
- Fast: Can be produced within hours
- Predictable: Small size distribution (2-8 nm) without agglomeration
- Catalysis/Photocatalysis
- Dye-Sensitized Solar Cells
- Light Emitting Diodes
SD#10638
Published12/6/2011
Last Updated5/20/2013