Miniature Acoustic Wave Lysis System (mALS)
Gaining timely and efficient access to target biomolecules through cellular lysis is a critical step in bioscience research and development. Researchers at Sandia National Laboratories developed a miniature acoustic lysis system (mALS) that uses high-frequency acoustic waves to rapidly disrupt cellular membranes for the release and extraction of DNA, RNA, and proteins without chemicals or additional processing. This portable system improves on commercially available lysing technologies with its rapid sample processing, minimal sample modification, condensed power and space requirements, and compatibility with high-throughput assays, nano/micro-fluidic and point-of-care devices. The mALS’ versatility and lysing effectiveness hold promise for rapid sample preparation in diverse settings, such as global health and research applications.
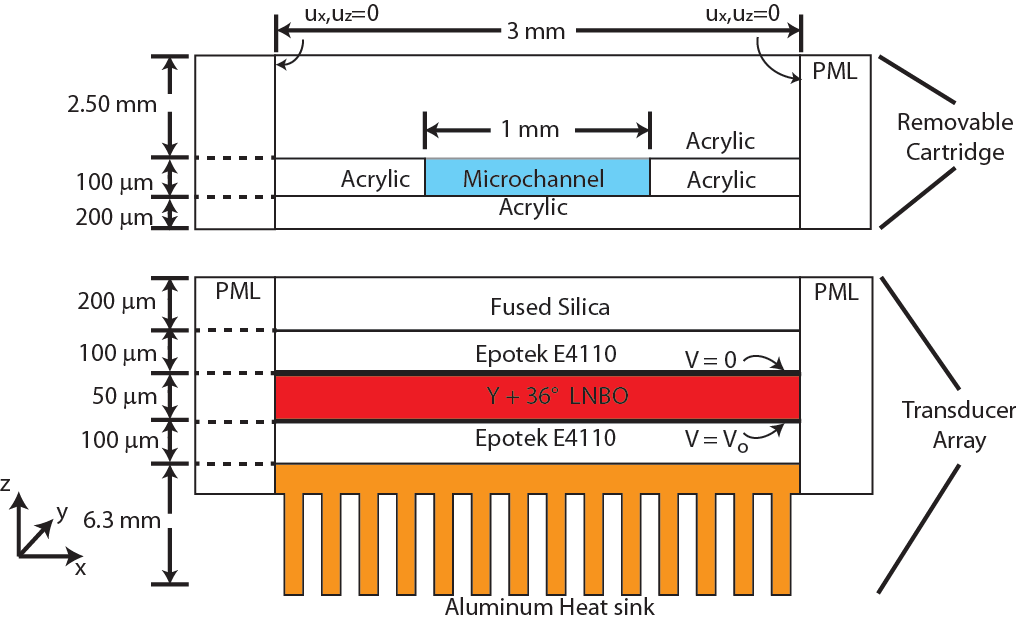
The system requires no external cooling and can operate under battery power or with a small switching power supply. Each element can efficiently and independently release genomic material or proteins rather than requiring multiple elements to process them. Compared to a bench-top acoustic lysis system, the mALS has been used to lyse E. Coli bacteria with a lysing dose (defined as ATP released per joule) 2.2x greater and with 6.1x more ATP released. An electric field-based nucleic acid purification approach using Nafion films yielded an extraction efficiency of nearly 70% in 10 min for 50 µL samples. The system consists of a compact, five channel array of miniature acoustic transducers and a disposable plastic cartridge that processes the cellular samples. The acoustic transducer array eliminates all thermal variation in contact with the plastic cartridges. Each acoustic transducer, fabricated using lithium niobate (LNBO), is driven by a radio frequency (RF) signal at 68 MHz coupled to a small 2W RF amplifier. The acoustic transducer has an area of 5 mm2 where the fluidic processing region scales as 1 channel for every 5.5 cm2 of cartridge space. The system requires no external cooling and can operate under battery power or with a small switching power supply. Each element can efficiently and independently release genomic material or proteins rather than requiring multiple elements to process them. Compared to a bench-top acoustic lysis system, the mALS has been used to lyse E. Coli bacteria with a lysing dose (defined as ATP released per joule) 2.2x greater and with 6.1x more ATP released. An electric field-based nucleic acid purification approach using Nafion films yielded an extraction efficiency of nearly 70% in 10 min for 50 µL samples.
- High throughput: Nearly 70% extraction efficiency in 10 minutes for 50 µL samples
- Increased effectiveness: Releases 6.1x more ATP than current bench-top acoustic lysis systems
- Integration with nano/microchannel devices
- Improved safety and biohazard containment
- Reduced cost, power, and space requirements
- Biotechnology
- Pharmaceuticals
- Infectious disease monitoring
- Genetic analysis
- Global health
- Microsensors
SD# 12600
Published10/28/2019
Last Updated10/28/2019